AstraZeneca Acknowledges Vaccine Side Effects in Isolated Instances
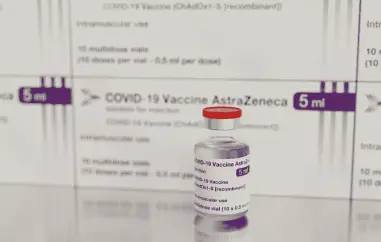 In a recent development, AstraZeneca has made a noteworthy concession regarding potential rare side effects associated with its COVID-19 vaccine, Vaxzevria. This acknowledgment, disclosed in court documents, could mark a significant juncture for affected individuals and their families, who have pursued legal action against the pharmaceutical company for compensation. Initial coverage of this revelation came from the British newspaper "The Telegraph."
In a recent development, AstraZeneca has made a noteworthy concession regarding potential rare side effects associated with its COVID-19 vaccine, Vaxzevria. This acknowledgment, disclosed in court documents, could mark a significant juncture for affected individuals and their families, who have pursued legal action against the pharmaceutical company for compensation. Initial coverage of this revelation came from the British newspaper "The Telegraph."
Ongoing litigation against AstraZeneca is currently underway in the High Court of London, stemming from a collective lawsuit. Since last year, the court has received 51 cases related to this matter. It is estimated that the plaintiffs and their relatives are seeking damages totaling up to £100 million. Allegations against the pharmaceutical company attribute deaths and severe impairments to its vaccine.
According to reports from "The Telegraph," court documents reveal that AstraZeneca admitted in response to one plaintiff that its vaccine "can cause TTS in very rare cases." However, the causal mechanism behind these occurrences remains unknown. Subsequently, in a letter to the plaintiff's legal representatives, AstraZeneca refuted any generalized assertion that its vaccine causes severe side effects, stating that the company does not "acknowledge that TTS is caused by the vaccine on a general level."
The potential risks associated with AstraZeneca's vaccine, particularly concerning younger women, came to light in March 2021, shortly after its provisional approval. Evidence emerged indicating that Vaxzevria could lead to thrombocytopenia and thrombosis in rare instances, collectively referred to as TTS within medical circles.
Thrombocytopenia involves a reduction in the number of platelets in the blood, heightening the risk of bleeding, while thrombosis entails the formation of blood clots that may obstruct blood vessels. Throughout vaccination campaigns administering the AstraZeneca vaccine to millions of individuals, fatalities resulting from TTS were reported.
Consequently, the Paul Ehrlich Institute issued its first Red Hand alert in April 2021, notifying medical professionals about safety concerns associated with the vaccine. AstraZeneca had previously indicated that a plausible connection existed between vaccinations with Vaxzevria and the incidence of TTS. Subsequent updates to these alerts consistently emphasized the rarity of thrombocytopenias and their tendency to manifest within the initial four weeks post-vaccination.
According to the Robert Koch Institute, 18 confirmed cases of TTS-related fatalities have been documented in Germany following AstraZeneca vaccinations. The risk appears to be particularly elevated among younger women, prompting the Standing Vaccination Commission to recommend the vaccine exclusively for older demographics. As per the Robert Koch Institute, its administration in Germany ceased in December 2021.
Image by Paul McManus from Pixabay